Articles
Engineers at the US Naval Research Laboratory (NRL) have produced jet fuel from seawater and then fuelled a model aircraft from it.
The researchers' process involved extracting the carbon dioxide and producing hydrogen from seawater, and subsequently converting them into jet fuel using a gas-to-liquids process.
The process is being developed to reduce the cost of refuelling at sea. In 2011, the US Navy Military Sea Lift Command, the primary supplier of fuel and oil to the US Navy fleet, delivered nearly 600 million gallons of fuel to Navy vessels.
The predicted cost of jet fuel using these technologies is in the range of $3-$6 per gallon, and with sufficient funding and partnerships, this approach could be commercially viable within the next seven to ten years, said NRL.
Fuelled by the liquid hydrocarbon, the research team demonstrated sustained flight of a radio-controlled P-51 replica, powered by an off-the-shelf and unmodified two-stroke internal combustion engine.
The fuel from seawater process uses an electrolytic cation exchange module (E-CEM). Both dissolved and bound CO2 are removed from the seawater at 92% efficiency by re-equilibrating carbonate and bicarbonate to CO2 and simultaneously producing H2. The gases are then converted to liquid hydrocarbons by a metal catalyst in a reactor system.
Dr. Heather Willauer, NRL research chemist, said: "In close collaboration with the Office of Naval Research P38 Naval Reserve program, NRL has developed a game changing technology for extracting, simultaneously, CO2 and H2 from seawater."
"This is the first time technology of this nature has been demonstrated with the potential for transition, from the laboratory, to full-scale commercial implementation."
CO2 in the air and in seawater is an abundant carbon resource, but the concentration in the ocean is about 140 times greater than that in air, and a third of the concentration of CO2 from a stack gas. Two to three percent of the CO2 in seawater is dissolved CO2 gas in the form of carbonic acid, one percent is carbonate, and the remaining 96 to 97% is bound in bicarbonate.
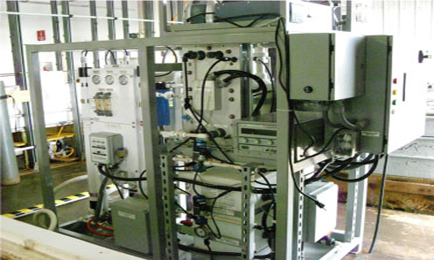
The machine used to extract hydrogen and carbon dioxide from seawater by US researchers
In the first patented step, an iron-based catalyst has been developed that can achieve CO2 conversion levels up to 60% and decrease unwanted methane production in favour of longer-chain unsaturated hydrocarbons. These value-added hydrocarbons from this process serve as building blocks for the production of industrial chemicals and fuels.
In the second step these olefins can be converted to compounds of a higher molecular using controlled polymerisation. The resulting liquid contains hydrocarbon molecules suitable for use a possible renewable replacement for petroleum based jet fuel.
Pursuing remote land-based options would be the first step towards a future sea-based solution, the NRL said.