Engineering news
US researchers have developed a fuel cell that also captures carbon dioxide.
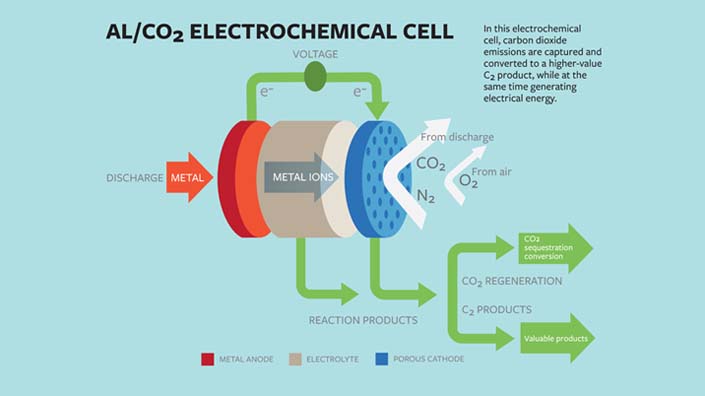
The oxygen-assisted aluminium/carbon dioxide power uses electrochemical reactions to both sequester the carbon dioxide and produce electricity.
The cell could be used to capture CO2 at power stations and vehicles and could use aluminium in its anode instead of higher cost, less safe materials such as lithium and sodium. It would use mixed streams of carbon dioxide and oxygen as the active ingredients of the cathode.
The electrochemical reactions between the anode and the cathode would sequester the carbon dioxide into carbon-rich compounds while also producing electricity and a valuable oxalate as a byproduct.
Lynden Archer, the James A. Friend family distinguished professor of engineering at Cornell University, said: “We’ve designed a carbon capture technology that also generates electricity.
“One of the roadblocks to adopting current carbon dioxide capture technology in electric power plants is that the regeneration of the fluids used for capturing carbon dioxide utilise as much as 25% of the energy output of the plant. This seriously limits commercial viability of such technology. Additionally, the captured carbon dioxide must be transported to sites where it can be sequestered or reused, which requires new infrastructure.”
The researchers said that the electrochemical cell generated 13 ampere hours per gram of porous carbon as the cathode, at a discharge potential of around 1.4 volts, comparable to that produced by the highest energy-density battery systems.
Archer said another key aspect of their findings is the generation of superoxide intermediates, which are formed when the dioxide is reduced at the cathode. The superoxide reacts with the normally inert carbon dioxide, forming a carbon-carbon oxalate that is widely used in many industries, including pharmaceutical, fiber and metal smelting.
“An oxalate that contains two carbons opens up a cascade of reaction processes that can be used to synthesise a variety of products,” he said.
Most carbon-capture methods capture carbon in fluids or solids, then compress and transport it to industries that reuse it, or sequester it underground. The findings in the study represent a possible paradigm shift, Archer added.
However, a drawback of the technology is that the electrolyte is extremely sensitive to water. Researchers are working on addressing the performance of electrochemical systems and the use of electrolytes that are less water-sensitive.
The paper, The O2-assisted Al/CO2 electrochemical cell: A system for CO2capture/conversion and electric power generation, was published during the summer in the journal Science Advances.