For a technology feted as having such a bright future, carbon capture and storage (CCS) is taking a painfully long time to emerge from laboratory-scale demonstration through to commercialisation. It was more than four years ago that the previous government identified the process as a highly promising technique that could help the UK to slash its emissions and achieve its 2050 climate change goals.
Subsequently, £1 billion was put aside to fund a project to demonstrate how the process would work in practice – capturing carbon dioxide from a fossil-fuel power station, transporting it by pipeline and storing it in an underground structure such as an oil and gas reservoir.
A protracted competition saw a scheme at the Longannet power station in Scotland emerge as the eventual winner. But this project was scrapped at the end of last year after concerns about costs, mainly to do with the length of pipeline required from the Fife coast to the North Sea oil fields where the carbon was to be pumped.
So we are now back to square one, with the Department of Energy and Climate Change prepared to say only that it will be launching an “accelerated” selection process “sometime soon”.
The procrastination is frustrating those who think that the UK, with its extensive expertise in the North Sea, is in a prime position to lead an industry that has enormous global potential. And there are fears that delays to the commercialisation of CCS could ultimately threaten the UK’s chance of meeting longer-term climate change commitments.
“The cancellation of Longannet was a real disappointment, and we have a history of several near misses on CCS projects in this country,” says Geoffrey Maitland, professor of energy engineering at Imperial College London, which has emerged as the central hub of CCS research.
“Even though the funding of just one project was a minimalist approach – we would have been better backing several horses – Longannet at least had been through a detailed assessment process and had buy-in from a large number of partners from the industry.
“The decision to cancel it was almost inevitable in terms of short-term economic drivers – the recession has come at the worst time for things like this. The decision puts CCS off for quite a few years, and the longer we put it off, the more CO2 we will have to capture to keep levels down.”
Maitland says that the delays in getting a large-scale CCS demonstrator up and running are particularly frustrating because, he believes, the UK has the geological conditions and the engineering knowhow to take a lead with the technology. That could create many thousands of jobs.
“There is the potential for the UK to get a large slice of the action,” he says. “Once CCS is proved commercially, there will be a domino effect, which means it will be retrofitted to existing power stations and installed on all newbuilds. There will be enormous business in building the capture technology and the pipeline structures, and the North Sea is a rich area in terms of storage capacity. Indeed, some small companies have already bought depleted oil and gas reservoirs and are waiting in readiness for this.”
While there are no large-scale demonstrations of CCS in the UK, that doesn’t mean that research isn’t ongoing into the techniques that underpin the process. Broadly speaking, there are three different capture technologies that can be fitted to fossil-fuel power stations: post-combustion, pre-combustion and oxyfuel (see box on page 36). Two of these – post-combustion and pre-combustion – can also be applied to industrial processes.
Capture accounts for two-thirds of the total cost of CCS, so much of the research and development effort is going into this area. The favoured technology at present is the use of amines as solvents to absorb large quantities of acidic CO2. The problem with this approach is that it’s extremely energy-intensive. Once the CO2 has been absorbed in the amine, a lot of energy is required to strip it out again.
Separation of the CO2 from other flue gases has to be done at low pressure. That requires compression to take it to the supercritical state, which is the most suitable form for transportation and injection. This process also adds significantly to the cost.
Quest for better solvents
So much effort is being applied to develop improved solvents that would absorb a lot of CO2 but require less energy-intensive processes to strip out the gas. And work is under way to develop processes that can be carried out at higher pressure, requiring less compression of the CO2 to get it to the supercritical state.
“We are looking at alternative amines, and mixing amines with other fluids such as hydrocarbon fluids, as they are much greener,” says Maitland. “Colleagues are also investigating ionic liquids, which are rather esoteric chemical cocktails that look very promising in terms of being able to create solvents with the combination of properties that we are looking for.”
Then there is a completely different approach which, instead of employing solvents, uses calcium or carbonate looping technology – essentially taking limestone and reducing it to calcium oxide. For capture purposes, the CO2 absorbs reactively on the calcium oxide, being converted to calcium carbonate.
In terms of release, the technique used is similar to that employed with amines. The calcium carbonate is heated and converted to calcium oxide, with the CO2 being transported and stored, while the calcium oxide goes back to capture more CO2.
“This technique has the potential to be more economic and have lower costs and higher efficiencies than the amine process but it’s less developed,” says Maitland. “It looks very promising, particularly in relation to the cement industry – the second-biggest producer of CO2 after the power generation industry – because calcium oxide is one of the raw materials in the clinker for cement.
“So Imperial is looking at linking this calcium looping process with cement manufacture: once the calcium oxide is spent and no longer efficient for CO2 absorption, it can
be fed in as a raw material for cement production. That gives it a double role, helping to bring down the cost of the overall process.”
After capture comes transportation, which would be a relatively simple process. The UK already has a gas infrastructure network, and it might prove possible to use part of that for CO2. However, CO2 is more corrosive than natural gas, presenting pipeline materials issues.
“I think that as this industry grows we will put in place a CO2 grid,” says Maitland. “This will help overcome issues of where the CO2 is captured in relation to where we will probably store it, in the North Sea. There are some issues to sort out with transportation, but they are not show stoppers.”
Storage does still present significant challenges, however, linked to the difference in geological conditions found in coal seams and sandstone and carbonate reservoirs. Carbonate reservoirs are usually fractured and therefore have a much wider range of porosity across the surface area. Furthermore, CO2 can react with the carbonate reservoirs, sometimes leading to precipitation as the supercritical gas flows through them over long periods. These differences present challenge in terms of understanding the processes and building the reactions into process simulations.
Imperial is one of the leading centres in the world when it comes to reservoir simulation. “To do these sort of calculations, we are dealing with CO2 mixed with hydrocarbon fluids and brines – and very little is known about the thermo-physical properties of these, the phase behaviour and the viscosity,” says Maitland.
“Our expertise includes measuring thermophysical properties of that sort under high-pressure and temperature reservoir conditions using specialised equipment that we have devised in-house. And we have experts in modelling of thermodynamic and transport properties under extreme conditions, which means that we can take molecular-based approaches that can be extrapolated over the wide range of fluid compositions and reservoir conditions.”
The next step on from the work that Maitland and his colleagues are carrying out at Imperial is to build larger-scale CCS demonstration plants that might, one day, bridge the gap between laboratory-based research and commercial projects of the size that had been planned at Longannet.
The most advanced example of this is a £21 million installation at the Ferrybridge power station in West Yorkshire, which went into operation last December (see illustration on page 32).
The project, which involves SSE, Doosan Power Systems and Vattenfall, employs post-combustion technology that captures 100 tonnes of CO2 per day from the equivalent of five megawatts of coal-fired generating capacity. It is the first demonstration plant of its size to be integrated into a live power station in the UK, and it will test the performance on real flue gas of the specific amine compound selected.
Link to larger plants
Mark Bryant, director of carbon capture at Doosan Power Systems, says that the significance of the project lies in its scale – at 100 tonnes per day it is two orders of magnitude larger than previous pilots. This enables the project to act as a bridge between smaller-scale demonstrators (around one tonne/day) and the larger plants (around 3,000 tonnes/day) envisaged by the government.
“This is the first trial to take previous work on small pilot plants and apply it to a much larger-scale facility. It will show us how this type of plant will perform when attached to the back of a conventional power station in its normal operating cycle. It will also give us an appreciation of longer-term operational issues so that we can build better reliability into new designs,” says Bryant.
Over the past few weeks, engineers at Ferrybridge have been undertaking the hot commissioning of the plant, running steam and flue gases into the demonstrator for the first time and checking the performance of the control systems. Bryant says the hot commissioning process has not presented any significant engineering issues, and that the plant will start its two-year test later this month.
Project partner SSE recently applied for European funding to build and operate a large demonstration plant based on post-combustion technology at Peterhead in Aberdeenshire. The results and operating experience from the project at Ferrybridge will feed into the Peterhead
project which, if funding is allocated, would come into operation in 2016.
So progress is evidently being made with CCS, albeit at a slower pace than was originally predicted. Looking into the future, Maitland at Imperial predicts that it will not be technical challenges that hinder the speed of adoption, but a lack of political will and collective action. He says that governments need to start acting collectively to push forward the technology.
“We already have the capability in capture and storage to make this technology work reliably,” he says. “But we need action on a pan-European scale. We need to get to
a tipping point where governments feel they can act collectively to provide economic and regulatory conditions where CCS can thrive. Whether that will happen any time soon remains to be seen.”
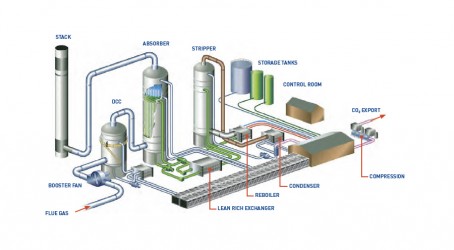
Scaling up at Ferrybridge
The Ferrybridge carbon capture trial in West Yorkshire, involving SSE, Doosan Power Systems and Vattenfall, uses a traditional post-combustion technology, working off a flue gas slipstream. An amine solvent is used to scrub the flue gas in a packed column, absorbing the carbon dioxide in the process. The solvent is heated to release the CO2 in a separate column and it is subsequently recycled back into the absorber.
As the Ferrybridge trial does not include a transport and storage element, the CO2 is then analysed for quantity and quality before it is reintroduced to the flue gas. In relative terms, the quantity of CO2 captured is small – 100 tonnes per day. It therefore wasn’t deemed practical to install compression, transport and storage elements. However, the project’s partners will be measuring CO2 production and composition. That in itself should deliver valuable data and demonstrate successful capture.
Choice of systems for power stations
- Carbon capture involves the production of a pure stream of carbon dioxide from a combustion process. There are three capture technologies that can be fitted to fossil-fuel power stations: post-combustion, pre-combustion and oxyfuel.
- In pre-combustion capture, a series of reactions is used to produce hydrogen (H2) and a pure stream of CO2 from a hydrocarbon fuel. The H2 can then be burned in, for example, a specially modified gas turbine, or a fuel cell.
- Post-combustion capture can be carried out in several ways. Solvent capture – including MEA or amine scrubbing – uses some form of solvent to reversibly react with CO2 from the flue gas after the fuel has been burned, with heat being applied to remove the CO2 and produce a pure stream of it.
- In oxyfuel combustion, the fuel is burned in pure oxygen (O2) – thereby forming a stream of pure CO2 and easily condensable steam – and some of the CO2 from the exhaust is recycled back to the combustor to moderate the flame temperature.
- Integrated gasification combined cycle, a pre-combustion technology, involves the gasification of the fuel by burning it in a small amount of O2 to provide a gas that is rich in carbon monoxide (CO) and H2. Further treatment produces separate streams of CO2, for sequestration, and H2, which can be burned without any climate change effects.
- The calcium looping cycle (post-combustion) uses the ability of calcium oxide (CaO), obtained by calcining natural limestone (CaCO3) to react with CO2 in the flue gas to reform CaCO3 – heating this regenerates the lime and again produces a pure stream of CO2.
- Chemical looping combustion uses a metal oxide to carry oxygen from air to a fuel, either gaseous or otherwise. The combustion of the fuel produces a pure stream of CO2. Direct carbon fuel cells and artificial leaves – to remove CO2 from the air – are also under investigation.