The British population may feel like it is generally under excessive surveillance, but the existence of the NHS means there is less of a call here than in the US for engineers to develop medical devices that can report back on patients’ behaviour.
Such medical technology – which can determine, for example, when, whether and how patients are taking drugs prescribed to them by doctors – is no longer the stuff of science fiction. And in North America, where insurers are acutely keen to clamp down on avoidable or excessive trips to the emergency room, mass-produced medical devices that also monitor patients are set to become widespread, say the experts.
“Insurers are interested in compliance – whether patients are complying with the medication regime. If they are not taking it correctly, there may be big hospital bills – a drain on your cover,” says James West, mechanical engineer and founder of Bristol’s Crux Product Design and engineering consultancy, which is developing this type of technology.
However, it’s not just a matter of “Big Brother watching you”, he says. Smart medical devices could also lead to technology that helps, say, sufferers from asthma to prevent an attack occurring, based on real-time information on local pollution levels. Monitoring the ways in which drugs are taken could also help train patients to administer them correctly.
But there is another aspect, says West. “If you end up in the emergency room with horrendous asthma attacks again and again, then the medicine is clearly not working for you as a patient – and you’d want to know why.”
Crux, which employs 13 engineers and designers at its Bristol base, regards itself as a medical engineering specialist. Much of its work is driven, like many others in the European medical device industry, by developments in the US. It’s commonly acknowledged that the regulatory regime there sets the pace, and that strictures that come into force “across the pond” will ultimately be felt in the rest of the developed world. “If it doesn’t work for the US market, doesn’t comply, then it’s accepted it’s not going to go,” says West.
The company is working on several devices that exploit the latest developments in miniature electronics to demonstrate smart medical technology to potential US customers.
This includes designs for an asthma inhaler that incorporate some of the wizardry found in consumer electronics. The low costs of wireless technology, display screens and power sources are allowing the manufacture of disposable medical devices that can upload data to the cloud, says West.
He demonstrates a design of inhaler that can effectively teach the patient how to use it. “That’s one of the benefits of this kind of technology,” he says. “With an asthma inhaler, you need to shake the canister for a given amount of time to prepare the dose. You need to mix the correct amount of drug and propellant. With an accelerometer onboard the electronics, you can monitor how long the patient has shaken the canister for and with buzzers give audible feedback: perhaps it beeps, and you’re ready to take the dose.”
There was a big stumbling block to overcome in the development of this smart asthma inhaler, he adds – its screen. The liquid crystal displays (LCDs) commonly used in other electronics devices are unsuitable for medical applications because of their use of glass, high power consumption, and relative level of robustness.
But a new type of plastic display has been developed by a British company with the help of the Technology Strategy Board, and this is being used on the curved surface of the inhaler. “The display can be curved with this technology – it doesn’t have to be straight-edged; it can have any footprint you want,” says West. “You just cut it out of a thin sheet – it’s almost like a label, and it’s cheap. You can compare it to the trademarked E Ink used on the Kindle. It also works in monochrome.” The technology is being readied for mass production, he says.

Bright idea: Thin sheet plastic display can be curved
The inhaler also has a wireless link to allow it to be connected to a PC. This means usage data can be uploaded to either personal or general medical records in the cloud. For instance, a device might be designed to dispense medication for one month, and to record details about drug usage for that period onboard the unit. The information would subsequently be uploaded by a doctor. Power usage would be kept to a minimum by only activating storage facilities on the device when a button is depressed to allow the medication to be dispensed.
It is thought the device could be used by asthma sufferers as a preventative measure, in tandem with mobile phone applications that report on levels on pollution or pollen count in the vicinity. “Effectively, you could decide to take a dose before you have an attack,” says West. Crux is also working on a smart insulin dispenser. The aim is to give diabetes sufferers an ultra-accurate method of dispensing the drug. “It’s easy to overdose or underdose, and that’s a problem.” The insulin device would report exact dosages.
Another innovative development from the company is mass manufactured syringes for the European market, where the latest safety requirements from North America have seen the development of syringes with retractable needles.
Many diabetics in the US still inject insulin with a traditional syringe rather than a modern, specifically designed insulin injector. This means sharps going into landfill. There are also concerns over sharps bins being compromised, exposing used needles to the outside world.
Crux’s engineers have developed a syringe design with a preloaded compression spring which sits on release clips. Once the drug has been delivered and the plunger has come to the end of its travel, the clips release and the needle is retracted by the spring back into the needle guard. The result is a syringe without a protruding sharp.
“It’s completely safe, and although not currently mandatory in Europe, our European customers have been working on a number of these projects,” says West. “They know it’s coming, and it’s too expensive to have two different production lines for each type of syringe, one for the US and one for Europe. They want to have one line making products for both markets.”
Designing the syringe required hundreds of hours of tests under varying loads and with simulations of dropping or misuse. Crux also developed a test version of the syringe, that could be given to doctors to demonstrate its operation for patients.

Safer syringe: Crux’s design for US needs…

…delivers the drug, its clips then release…
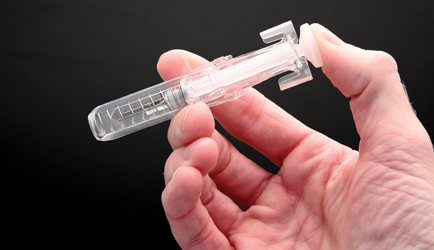
…and a compression spring retracts the needle
How does a business the size of Crux get work in the medical market? Many of its customers are massive pharmaceutical businesses, which may have an office in the UK or Germany – where Crux has a subsidiary (see box) – but operate globally, says West. These large multinational pharmaceutical and medical companies tend to outsource the design and engineering of drug delivery systems.
“They are focused on breakthroughs in drugs,” he says. “One of those means billions: compared with that, our fees are peanuts. But the companies don’t throw their money around.” Building a track record in the sector takes time, he adds, and word-of-mouth recommendation is important. The business will also attend trade fairs, including giant industry events such as Dusseldorf’s Medica, which takes place next month and will see the launch of the inhaler.
Ironically, Crux has recently been approached to develop designs for e-cigarettes, but West says this work does not interest the firm. “We won’t do defence work, either,” he says. “Medical is fascinating to work in: it’s methodical, and it can’t be rushed out of the door. And once you have devices out there, it means revenue over a number of years.
“Once you’ve got the reputation of delivering great ideas, great intellectual property and doing it in a timely manner, they [pharmaceutical companies] enjoy working with you. It is something engineers in the UK are good at. You have to be nimble enough to spot trends, and also take an interest in the latest technologies. And we’re continuing to sell great ideas.”
Delivering growth: Crux expands despite global financial crisis
Crux Product Design typically works on medical projects that have begun with a request from a customer to develop a delivery system for a drug. The business is owned by its directors and has never taken on any debt.
During the worst of the financial crisis, founder James West and his colleagues used profits that had been saved during the business’s early days to expand it.
“We’ve always had growth ambitions. We saved money for a rainy day, and in between 2008 and 2010 took the decision to expand,” he says.
During this period, the business went from two founders in Bristol to 13 engineers and designers. A further 13 engineers were taken on at the company’s German subsidiary, which had been developed in response to European demand. “Is it any easier to recruit engineers in Germany? I wouldn’t say so,” says West.
“A lot of the best engineers go into the automotive industry, so you compete against that. But the German engineers are great to work with: they are thorough, organised – and a lot of fun.”
West worked at HP before setting up Crux, and he prefers life in a small engineering firm. “But I understand the pressures project managers in multinational businesses are under – it’s not necessarily their fault that something is taking so long to do,” he says.
One of the crucial areas for the business in terms of its expertise is human factors.
While the firm keeps a close eye on regulatory developments in the US, it also responds to expectations from consumers that devices, as well as functioning effectively, should be easy to use and even aesthetically pleasing.
There is an greater level of consumer choice in the US market for medical devices, but even in Europe patients are more demanding.
“It’s important that a device feels good and intuitive to use: good design is critical,” says West.
“Also, you don’t want to be using a device that’s embarrassing to have out in public. You either need to be able to use it discreetly, or to have something that won’t look like some weird medical contraption on the dining-room table.
“It might be more akin to a mobile phone, or something like that.”