Technology with a buzz: The Sting device's 'programmable bevel' tip mimics the movement found in certain wasps
Engineers and scientists have long taken inspiration from nature to create elegant solutions to life’s challenges. From Velcro tape inspired by the hooks on a burr seed to aircraft ‘winglets’ that mimic the upward curl of an eagle’s feathers, there is a long and eclectic list of technologies developed over the past 50 years that have been modelled on natural mechanisms.
Some of the world’s leading biomedical engineers are once again looking to nature to help them solve pressing healthcare issues. Mimicking the solutions nature has refined over millions of years has enabled engineers to break new ground in areas as diverse as medical robotics and the early detection of illness.
Researchers at Imperial College London are often leading the way in these efforts. Molly Stevens, professor of biomedical materials and regenerative medicine at Imperial, has gained widespread internatonal recognition for her work in developing materials that take inspiration from biology. Stevens runs her own multidisciplinary team of researchers that, thanks to its pioneering work, has been named Research Group of the Year in the European Life Science Awards. The Stevens Group consists of engineers, chemists, biologists, physicists and surgeons who work together to create bio-inspired materials to treat some of the world’s biggest killers, including cancer and heart failure.
Stevens says: “There are so many reasons why research in regenerative medicine is beneficial for society. There are many diseases that result in damaged tissues, and the goal of regenerating failing organs, before the body as a whole is ready to surrender, is now timelier than ever.”
Rather than try to ‘out engineer’ nature to solve health problems, Stevens believes biology already provides the perfect blueprint. “Nature continues to inspire our designs and instructs on the most elegant construction of materials,” she says. “It is remarkable how far we have come in our ability to mimic natural constructs but we still have so much to do before we can truly say we meet nature’s standards.”
The group’s attempts to meet these high standards have resulted in novel approaches towards tissue regeneration, including the design of artificial nano-scaffold structures that mimic the nanostructure of the tissues in the body, on which new cells are encouraged to grow. This innovative approach is capable of producing new tissue with a more native like structure.
Stevens has made enormous advances in her group at Imperial in developing materials for regenerative medicine and ultrasensitive biosensing. She also had a ‘eureka’ moment during her postdoctoral research at Massachusetts Institute of Technology, working with a number of important collaborators including Prasad Shastri and Robert Marini in the laboratories of Professor Robert Langer – often hailed as the founding father of tissue engineering.
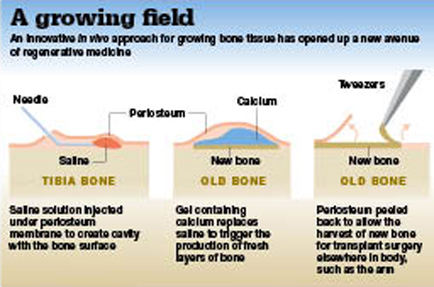
They co-developed an injectable alginate hydrogel and utilised it in an ‘in vivo bioreactor’ approach. Naturally occurring sodium alginate is found in certain types of brown seaweed. In its extracted form it is capable of absorbing 200-300 times its own weight in water and forms a gel in the presence of calcium.
To regenerate bone in vivo, says Stevens, the alginate hydrogel is implanted between an outer stem-cell-rich layer, called periosteum, and underlying bone. “This then acts as an artificial space, or scaffold, for cells to infiltrate and proliferate and grow high-quality new bone, which can then be easily removed and transplanted elsewhere,” she says. The technique is moving closer to clinical use, and Stevens envisions a future where clinicians will be able to use it to generate “bone on demand”.
The Stevens Group has since designed new-generation hydrogels for other tissues, including cartilage and cardiac applications. Such biomimetic solutions have brought the field of biomedicine forwards in leaps and bounds. Stevens is anything but complacent, though: “The field has made significant progress in exploiting natural processes for healthcare applications, but there is still much to be done, especially for tissues that have generally less regenerative capabilities, such as cartilage, cardiac and neural,” she says.
Mimicking nature has also proved an incredibly useful tool for the advancement of medical robotics. Dr Patrick Finlay, founder of medical robot development company MediMaton and chairman of the IMechE Biomedical Engineering Association, says that taking examples from the animal and natural world is a logical step to drive progress: “When you’re working on the human body, which is biological, it makes sense to use biological structures because rigid robot arms will not work when dealing with the kind of shapes and curves of the human physiology.”
Many first-generation medical robots, Finlay says, were bulky machines based on industrial arms. Large numbers of these are still in daily clinical use. “Conventional existing medical robots can only move in straight lines and have to be steered by a surgeon from the outside, which has some limitations when trying to navigate up a blood vessel or trying to find somewhere deep inside the brain without going through or damaging vital areas.”
However, by taking a leaf out of nature’s book, second-generation robots are becoming smaller and are able to carry out procedures that simply would not be achievable with the human hand or eye.
One such robot is called Sting – Soft Tissue Intervention and Neurosurgical Guide. It is being developed by Dr Ferdinando Rodriguez y Baena at Imperial College as part of a project with the same name, founded by the European Research Council (ERC). It provides a way of steering a long, flexible probe into the brain by means of a "programmable bevel" tip inspired by the structure and mechanisms of the ovipositor (egg-laying channel) of certain parasitic wasps.
The ovipositor is composed of two or more segments (valves) which are connected by means of a special dovetail interlocking mechanism and can therefore slide with respect to each other. Finlay says: “The clever bit is if you push one needle further than the other it will start to go in a curve. And if you want to move it in the third dimension, a minimum of three segments suitably offset with respect to each other will do the trick.” This could be used for the treatment of tumours, whereby the device would deliver tiny doses of therapeutic agent at precise points around it, deep inside the brain. This would save countless hours, Finlay says, and is much more precise than surgeons effectively operating ‘blind’ outside the brain.
Other significant advances in surgical procedures are being made around the world thanks to biomimetic medical robots, from flagella-propelled nano robots designed for eye surgery to swallowable capsule cameras that are able to stick to the wall of the gut by mimicking the adhesive foot pads of the palmetto beetle.
Finlay believes that biomimetic medical robots are the future of surgery. But he adds: “It is not an area that is coming together yet. There are lots of people digging holes in different places, but that’s what normally happens in a new field and it’s very exciting to see.”
Professor Christofer Toumazou, founder and chief scientist of the Institute of Biomedical Engineering at Imperial College, has been digging away at a particularly exciting area of research. With a background in developing low-power analogue chips for mobile phones, he now uses the same innovative technology to create methods of monitoring, treating and diagnosing medical conditions, as well as supporting or augmenting failing organs.
His pioneering work came about through a fundamental realisation that biology is analogue. Toumazou explains: “We don’t see with 20 bits of precision, we don’t hear with 20 bits of precision – biology just doesn’t work with the precision of the digital world. People have tended to ignore that over the years and focused on digital solutions instead of analogue ones.”
Toumazou says that he simply looks at biology as if it is one big equation. He then mimics those equations on hardware, not software, to replace the function of the faulty biology in the body, opening up a whole new domain of bio-inspired technology.
In 2003 Toumazou first successfully utilised his analogue microchip technology with a Canadian company, Epic Bio Sonics, to create an implantable cochlear for children who are born deaf. They had a miniature electrode array they could implant in the ear, says Toumazou, but were using a gigantic digital processing chip outside the brain to mimic the behaviour of the cochlear to send the right electrical signals to the brain.
He says: “I took this entire processing unit that they did digitally and were trying to do with 20 bits of precision, and I was able to replicate it with a small, few nanometres microchip that could be completely implanted in the ear and powered up with an inductive loop. With just 16 electrodes, the brain had enough plasticity and regeneration capability to give a deaf child hearing. It was wonderful to see how the brain adapts with information that doesn’t have to be precise.”
Toumazou has since gone on to apply this technology to create an artificial pancreas for type-one diabetics, an intelligent neural stimulator as a drug alternative for treating obesity, and a ‘digital plaster’, or Sensium pad, that can be stuck to a patient’s chest to wirelessly monitor heart rate, respiration, ECG signals and temperature.
He has gone on to create microchip technology that can respond to a human’s chemistry rather than electrical impulses, breaking new ground in the field of early diagnostics. Toumazou says: “I went back to basics and said OK let me put DNA on the microchip, and I think I had the biggest eureka moment of my whole academic career – it turned on the microchip.”
This revelation has led to the invention of a tiny analogue device called the Genealysis chip. This USB-like chip can analyse a person’s DNA within 30 minutes, with no need for a lab. Toumazou says: “The key applications for me were to target particular health issues that people would be looking for answers to, such as type-two diabetes, or whether they can metabolise a certain kind of drug.”
Each chip, he explains, has an imprint or primer of the target DNA it is designed to detect, such as type-two diabetes, and tests an individual’s DNA for the target by mimicking a biological process called polymerase chain reaction. The implications, he says, are huge, with the technology having the potential to be used in personalised medicine and the detection of super bugs in hospitals.
From building bone to bio-inspired microchips, the possibilities of applying biomimetics to medicine are far reaching and have the potential to transform the way we treat illness.
However, Stevens, Finlay and Toumazou agree that there are many barriers to getting these treatments into our healthcare system, from lack of funding and strict regulations to the traditional attitudes of medical professionals.
For Finlay, though, it is an inevitable process that will see the inclusion of nature’s elegant solutions in medical technology that will far surpass our current methods. He says: “Future generations of doctors will think it’s highly amusing that in the early 21st century we’d take a knife and cut patients open and stick our fingers inside and pull things out – it’s just crude.”
Get down to Bond Street for beauty on a chip
Christofer Toumazou’s company Geneu has created a “beauty lab on a chip” that can provide customers with personalised anti-ageing skincare products matched to their DNA. Geneu’s flagship store in London’s Bond Street is set to open next month.
Company director Dr Martin Stow says: “The synergy between molecular biology, innovative technology and the skincare industry represents a breakthrough in consumer point-of-care DNA testing and the personalisation of skincare.” Toumazou says: “The whole objective with Geneu is to take the stigma away from it being a medical device, to beautify medical technology.”